(NH4)2SO4/C4H4O4/H2O Ternary System
Abstract:
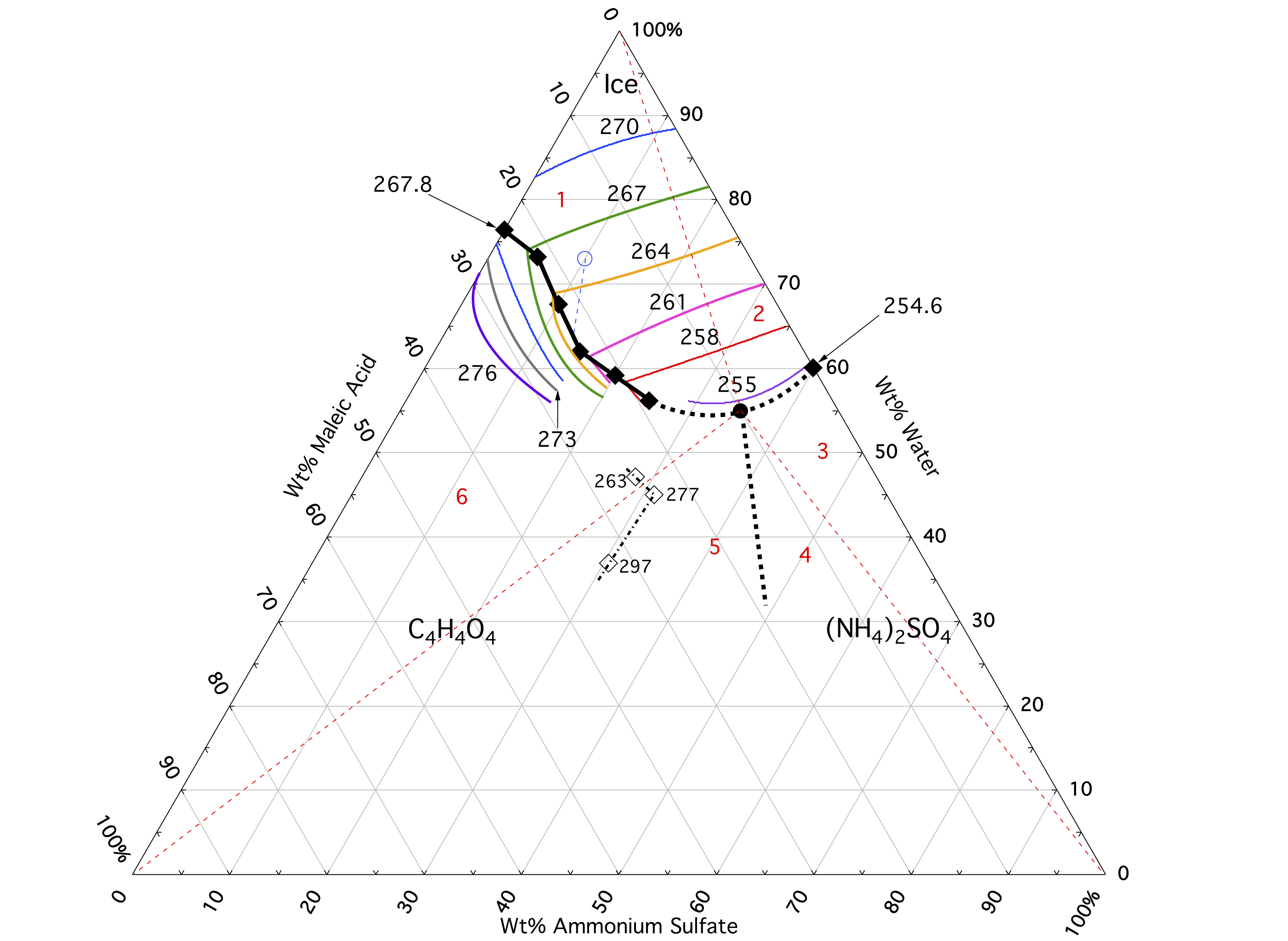
We have studied the low temperature phase diagram and water activities of the ammonium sulfate/maleic acid/water system using differential scanning calorimetry, and infrared spectroscopy of thin films. Using the results from our experiments we have mapped the solid/liquid ternary phase diagram, determined the water activities based on the freezing point depression, and determined the ice/maleic acid phase boundary as well as the ternary eutectic composition and temperature. We also compare our results to the predictions of the Extended AIM Aerosol Thermodynamics Model (E-AIM), and find good agreement for the ice melting points in the ice primary phase field of this system; however significant differences were found with respect to phase boundaries, maleic acid dissolution, and ammonium sulfate dissolution.
Read the Paper Here