H2SO4/H2O Binary System
This project was completed while the Beyer research group was located at Wisconsin Luthern College.
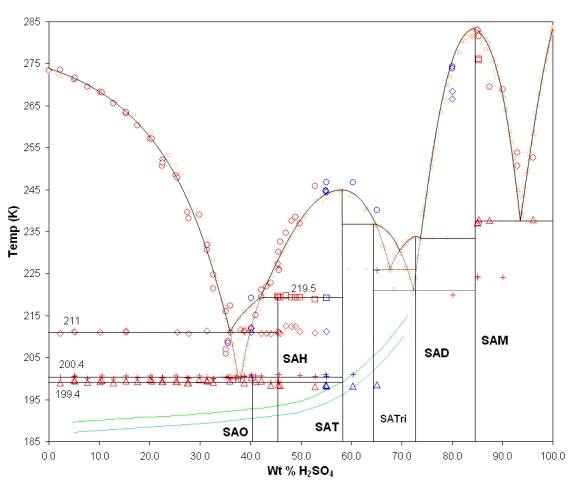
Abstract: We have investigated the H2SO4/H2O binary liquid/solid phase diagram using a highly sensitive differential scanning calorimeter (DSC) and infrared spectroscopy of thin films. In particular we have sought to investigate the region from pure ice to sulfuric acid tetrahydrate (SAT), including a detailed look at the sulfuric acid octahydrate (SAO). Our studies have found that there is a unique, repeatable IR spectrum for SAO. Our spectrum is not merely a combination of spectra of ice and sulfuric acid tetrahydrate (SAT), as has been previously suggested could be the case by Zhang et al. (Zhang, R.; Wooldridge, P. J.; Abbatt, J. P. D.; Molina, M. J. J. Phys. Chem. 1993, 97, 7351). From our thermal analysis experiments, we have identified the melting transition of SAO. We report for the first time in the literature the enthalpy of fusion of SAO. We have also determined from our studies using the energies of fusion of ice and the various hydrates of H2SO4 that SAO is a major component of H2SO4 solutions in the range 20-40 wt % when they freeze. Our results indicate that SAO could be present in solid or partially frozen polar stratospheric and upper tropospheric cloud particles. Finally, we make a comment based on our results regarding the stoichiometric composition of SAO.
Read the Paper Here