
Adrienne Loh (UW-L) & Robert Oswald (Cornell University)
Protein Dynamics:
Protein structures are not static. Many of the bonds in a protein can rotate and flex, and entire structural segments of the protein can move on a variety of timescales. The types and timescales of motion that the protein experiences can play a significant role in the way that the protein functions. For example, some proteins such as the Ras-like GTPases are capable of interacting with a variety of different proteins using the same binding surface (1-6). This suggests that some proteins must have binding regions that are able to adjust to a variety of different incoming ligands.
In a collaboration with Dr. Robert Oswald at Cornell University, we are studying the dynamics of the G-protein Cdc42Hs, a protein is involved in a variety of cytoskeletal and growth functions. Cdc42Hs acts like a molecular switch that is off (inactive) when a molecule of GDP is bound, and on (active) when a molecule of GTP is bound. When active, Cdc42Hs can bind a variety of other proteins, in turn switching them on, thus initiating a functional cascade.
The dynamics of protein motion can be characterized using multidimensional NMR spectroscopy (xx). NMR spectroscopy is a particularly useful tool for investigating protein motions because it is sensitive to molecular motion over a wide variety of timescales. In one such study, we characterized the dynamics experienced by the backbone nitrogen atoms in three constructs of 15N-labeled Cdc42Hs (inactive, active, and active with a bound protein fragment) by measuring several relaxation parameters using 15N-1H NMR spectroscopy. This data yielded information on the type and timescale of backbone motions experienced by Cdc42Hs (Figure 1). Overall, significant flexibility was observed mainly in the regions of Cdc42Hs that are involved in protein-protein interactions. This flexibility was reduced upon interaction with a protein ligand, indicating that segmental flexibility is essential for high affinity binding interactions.
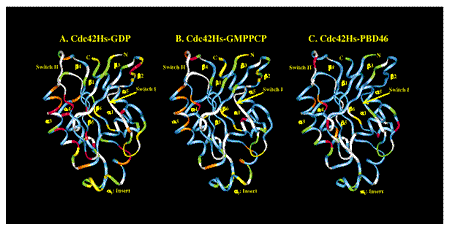
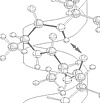
Figure 1: Best fit models for the dynamic behaviors of the backbone nitrogen atoms in the three constructs of Cdc42Hs . White: unassigned residues; Blue: model 1 (simple motion on a very fast timescale); Green: model 2 (simple motion on moderately fast timescale); Yellow: model 5 (complex motion on two timescales); Orange: model 3 (simple motion on a very fast timescale, and motion between two or more distinct chemical environments); Red: model 4 (simple motion on a moderate timescale, and motion between two or more distinct chemical environments).
We have also analyzed the dynamics of the sidechain methyl groups in the same forms of Cdc42Hs using 13C-1H NMR spectroscopy (xx). We found that while the motions of the on (GDP-bound) and off (GTP-bound) forms are similar, binding of a protein ligand results in a significant increase in the disorder and a corresponding increase in entropy for the majority of methyl groups (Figure 2). Many of these are found in residues that are not part of the ligand binding interface. This increase in mobility represents a gain in entropy that helps to partially offset unfavorable entropy losses such as those that occur in the backbone. This is an exciting and interesting result, which indicates that protein function (initiated by the spontaneity of a process like binding) involves a delicate balance between entropy and enthalpy changes, and that unfavorable changes can be "made up" in distant locations from the binding site.
We are currently pursuing temperature-dependent studies of the backbone dynamics in order to learn more about the behavior of this protein and hopefully discover more about how entropy influences function.
Figure 2: Best fit models for the dynamic behaviors of the backbone nitrogen atoms in the three constructs of Cdc42Hs . White: unassigned residues; Blue: model 1 (simple motion on a very fast timescale); Green: model 2 (simple motion on moderately fast timescale); Yellow: model 5 (complex motion on two timescales); Orange: model 3 (simple motion on a very fast timescale, and motion between two or more distinct chemical environments); Red: model 4 (simple motion on a moderate timescale, and motion between two or more distinct chemical environments).
References:
1. Rittinger, K., Walker, P. A., Eccleston, J.
F., Nurmahomed, K., Owen, D., Laue, E., Gamblin, S. J., and Smerdon, S.
J. (1997) Nature 388, 693-607.
2. Nassar, N., Hoffman, G. R., Manor, D., Clardy, J. C., and Cerione,
R. A. (1998) Nature Struct. Biol. 5, 1047-1052.
3. Hoffman, G. R., Nassar, N., and Cerione, R. A. (2000) Cell 100, 345-356.
4. Guo, W., Sutcliffe, M. J., Cerione, R. A., and Oswald, R. E. (1998)
Biochemistry 37, 14030-14037.
5. Abdul-Manan, N., Aghazadeh, B., Liu, G. A., Majumdar, A., Ouerfelli,
O., Siminovitch, K. A., and Rosen, M. K. (1999) Nature 399, 379-383.
6. Mott, H. R., Owen, D., Nietlispach, D., Lowe, P. N., Manser, E., Lim,
L., and Laue, E. D. (1999) Nature 399, 384-388.
7. Loh, A. P., Guo, W., Nicholson, L., and Oswald, R. E. (1999) Biochemistry 38, 12547-12557.
Loh Homepage UWL Homepage